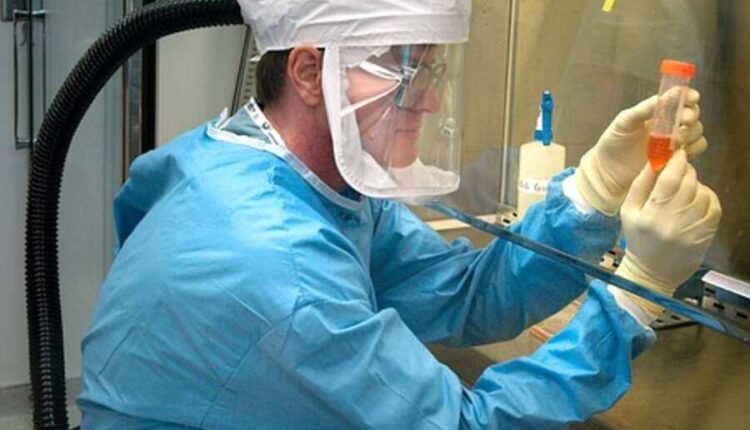
STERIS provides infection prevention and sterilization solutions to healthcare organizations worldwide. Their products and services help customers avoid HAIs (hospital-acquired infections) while improving patient safety and outcomes.
Applied Sterilization Technologies’s segment offers contract sterilization and decontamination services using gamma radiation, electron beam irradiation, and ethylene oxide technology. Furthermore, this company provides products and services related to sterile processing and instrument management.
Gamma Sterilization
Gamma sterilization is a non-thermal process that uses radiation to destroy microorganisms on a molecular level. Breaking bacteria’s DNA covalent bonds (remember organic chemistry class?) inhibits their growth and reproduction, resulting in sterilization. Gamma radiation has been successfully utilized over four decades with full regulatory approval, making it a preferred method for mass sterilization of medical devices, polymeric medical devices, food samples, etc.
Gamma radiation stands out among other irradiation technologies by its ability to penetrate different types of materials, products, and packaging – including dense items like pallets. Because of this versatility, it makes gamma the ideal technology for bulk sterilization applications.
Gamma radiation is also a cold process, meaning it does not rely on elevated temperatures and, therefore, cannot affect product performance or quality. Furthermore, unlike EtO and formaldehyde treatments, which interact with plastics differently, this factor makes gamma treatment ideal for medical device manufacturers that utilize polymeric materials.
Gamma sterilization remains the primary solution for medical device sterilization worldwide and holds the largest market share. Gamma remains an essential part of healthcare supply chains around the globe and is well positioned to meet increasing population needs as people live longer lives.
STERIS provides end-to-end sterilization solutions, laboratory testing, and advisory services for customers across the healthcare, life sciences, and dental industries. Utilizing cutting-edge technologies, we help create a healthier and safer world, including consumables, equipment maintenance and specialty services, and capital equipment primarily targeting medical device and pharmaceutical customers.
Steris offers a comprehensive portfolio of sterilization technologies, including gamma ray, electron beam (e-beam), X-ray, and ethylene oxide (EO) sterilization methods. Together these techniques ensure complete product contamination protection during manufacturing, as well as high-quality medical treatments and pharmaceuticals delivered safely and efficacy to patients. With such advanced capabilities and an expert team, STERIS is your one-stop solution for meeting all your sterilization needs.
Electron Beam Sterilization
Electron beam sterilization (E-beam) is an efficient sterilization solution for heat-sensitive medical devices, utilizing beta radiation in the form of high-energy electrons to penetrate microorganisms’ RNA/DNA chains and disrupt them, effectively eliminating their ability to survive and proliferate. E-beam does not cause degradation to medical products during its process and may be done while they remain packaged as intended.
E-beam sterilization is an automated process requiring minimal human involvement, ideal for sterilizing devices of various shapes and sizes ranging from small packages to large boxes or pallets. Furthermore, this approach eliminates costly breathable packaging solutions, saving costs and time.
STERIS plc generates revenue not only through sales of equipment and materials but also from rental/leasing of its equipment and income from spare part sales; additionally, it earns income through sterilization services.
STERIS plc operates four business segments, Healthcare Products, Healthcare Specialty Services, Life Sciences, and Applied Sterilization Technologies. Their products and services help protect patients in healthcare environments while supporting research and development activities.
This company provides comprehensive sterilization and infection prevention solutions to healthcare facilities and pharmaceutical and biotechnology industries worldwide, helping ensure patient safety.
This company stands out in the healthcare industry by its multiple revenue streams, global presence, and diverse business model, contributing to strong financial performance over time. Currently, the focus is on building leadership positions within key markets while further diversifying earnings with product and service offerings to bolster competitive advantages within healthcare. Innovation remains at the core of their growth strategy, ultimately leading to improved customer satisfaction and more revenues for the company.
Ethylene Oxide Sterilization
Ethylene oxide (EO / ETO) sterilization is a gaseous process that uses oxygen-enriched air to destroy bacteria, viruses, and fungi by forcing their metabolism into pathways that prevent their continued growth. It’s used extensively to sterilize medical devices, plastics, and cellulose products, which cannot be fixed by heat or radiation, such as medical devices. Our TechTeam specialists at STERIS strive to offer our clients the highest level of service when creating and optimizing ethylene oxide sterilization cycles for our client’s benefit.
EO cycles are ideal for thermosensitive products that cannot be sterilized through conventional sterilization methods such as steam and dry heat, complex plastic components with non-smooth surfaces, and natural or synthetic fabrics such as latex gloves and catheters. They’re also effective at sterilizing medical devices with long lumens, such as endoscopes.
Sublimation is a low-temperature process, typically within the range of 30degC to 60degC. It penetrates porous materials such as plastic and rubber to access all product areas – particularly handy when dealing with difficult-to-reach locations like creases.
The success of the ethylene oxide sterilization process lies in its ability for gas molecules to spread throughout a product and its packaging and simultaneously control four interdependent variables: concentration, temperature, relative humidity, and exposure time.
Ethylene Oxide Sterilization Technology boasts an incredibly high penetration rate into plastic and rubber materials and their pores. Furthermore, its sterilization cycle can reach hard-to-access creases and other tight spaces without deforming or disfiguring them – with no deformed products produced due to treatment! Moreover, this method can even be used on metals and woven products when packaged with breathable packaging, enabling penetration by the EO.
This process must be executed safely as a highly toxic, flammable gas. Individuals involved with sterilization techniques such as EO require personal protective equipment – gloves and a face mask are mandatory – when working in these facilities; air monitoring has revealed higher rates of Hodgkin’s lymphoma and breast cancer than their peers in nearby communities.
X-Ray Sterilization
The STERIS Applied Sterilization Technologies segment provides contract sterilization, laboratory testing, product and packaging testing services, equipment maintenance services, specialty services, and capital equipment to medical device and pharmaceutical manufacturers in the US and beyond. It generates most of its revenue in this country and represents its most significant revenue and profit segment; they focus on organic growth and inorganic strategies, such as acquisitions, to maintain its market position while expanding its business.
X-ray sterilization uses high-energy electrons to generate a sterilizing dose of radiation that penetrates deeply into materials without producing breakdown products. It can be applied to products of all densities, sizes, and thicknesses without having breakdown products; and is one of the fastest sterilization techniques. X-rays can quickly sterilize liquid, gaseous, and solid materials, thus being useful as single-use devices and manufacturing materials in production lines.
This sterilization method offers a high sterility assurance level (SAL) of 10-6 or better, meaning that less than one out of every million microorganisms survive. This approach offers an efficient alternative to hot steam treatment, which has the potential to degrade polymers while creating supply-chain disruptions due to environmental concerns and EtO capacity constraints.
As cobalt-60 costs continue to skyrocket and regulatory agencies reevaluate EtO, there has been increased interest in alternatives like ionizing radiation-based modalities such as e-beam and X-ray radiation technologies that use similar radiation sources. Over the last decade, these technologies have significantly advanced, becoming readily available industrial-sized equipment for both. ISO 11137 considers both modes equivalent, requiring similar material activation testing processes to achieve comparable sterility results.
Though X-ray may offer many advantages over its competitors in sterilization processes, companies may be reluctant to explore it as an alternate solution due to a lack of understanding about the process or fear that requalifying products for X-ray might delay production. Our Team Nablo initiative can bridge this gap and give confidence for a smooth transition from gamma sterilization to X-ray for metal product groups.